| GAMESS/Chem3D
calculations |
Experimental values |
Reference |
|
|
|
|
|
|
|
|
|
|
| ------------ Property Broker
------------ |
|
| Cp = 19.173 cal/(mol K) |
30.404 |
NIST |
|
| Cv = 19.173 cal/(mol K) |
N/A |
|
| Entropy = 74.46 cal/(mol K) |
34.419 |
NIST |
|
| Dipole = 0 Debye |
N/A |
|
|
| Boiling Point = 443.214 Kelvin |
455.02 |
CRC |
|
| Critical Pressure = 59.263 Bar |
N/A |
|
| Critical Temperature = 677.453 Kelvin |
694.2 |
CRC |
|
| Critical Volume = 279.5 Cm^3/Mol |
N/A |
|
| Gibbs Free Energy = -32.94 KJ/Mol |
-32.63 |
CRC |
|
| Heat Of Formation = -96.48 KJ/Mol |
-96.4 |
CRC |
|
| Henry's
Law Constant = 4.704 |
N/A |
|
| Ideal Gas Thermal Capacity = 99.577
J/(Mol.K) |
103.22 |
NIST |
|
| LogP = 1.477 |
N/A |
|
| Melting Point = 282.5 Kelvin |
314.04 |
CRC |
|
| Mol Refractivity = 27.752 Cm^3/Mol |
N/A |
|
| Vapor Pressure = 4.758 Pascal |
5.93 |
CRC |
|
| Water Solubility = 82800 Mg/L |
83000 |
CRC |
|
| Full Report: |
|
|
| ************************************************************************ |
|
| Data from database |
|
| ************************************************************************ |
|
|
| <Name of
molecule>>HOUSE,M.W.,BIOCHEM.PHARMACOL.,14,547(1965) |
|
|
| THIS VALUE APPEARS "OUT OF
LINE" &T.J.PHARMACEUT.,6,339(1980) |
|
|
| 0.62 |
|
|
| <DO = DENSITY OF ORGANIC SOLVENT
ANDMWO = MOLECULAR WEIGHT OF ORGANIC SOLVENTEXPRESSION HOLDS ONLY FOR LOW
CONCENTRATION OF SOLUTE |
|
| -0.78 |
|
|
|
< Chim.Theor.,19,71(1984). |
|
|
| ************************************************************************ |
|
| Estimation of Molar Refractivity |
|
| ************************************************************************ |
|
|
| MR............: 27.09 [cm.cm.cm/mol] |
|
| St..deviation.: 1.27 |
|
| by
Crippen's fragmentation: J.Chem.Inf.Comput.Sci.,27,21(1987). |
|
|
| MR............: 27.75 [cm.cm.cm/mol] |
|
| St..deviation.: 0.77 |
|
| by
Viswanadhan's fragmentation: J.Chem.Inf.Comput.Sci.,29,163(1989). |
|
|
| ************************************************************************ |
|
| Estimation of Henry's Constant (H) |
|
| ************************************************************************ |
|
|
| 1. method: H = 4.640 log[unitless] |
|
| Estimation of mean error..:
0.340 |
|
|
| 2. method: H = 4.704 log[unitless] |
|
| Estimation of mean error..:
0.200 |
|
|
| ************************************************************************ |
|
| Estimation of the Boiling and Freezing
points. |
|
| ************************************************************************ |
|
|
| Normal Boiling Point [p=1atm]: 443.21
[K] |
|
| Standard Error: 20.400 [K] |
|
| Joback
fragmentation method modified by S.E. Stein |
|
|
| Normal Boiling Point [p=1atm]: 439.20
[K] |
|
| Standard Error: Error was not estimated. |
|
| Joback
fragmentation method |
|
|
| Freezing Point [p=1atm]: 282.50 [K] |
|
| Standard Error: 25.000 [K] |
|
| Joback
fragmentation method |
|
|
| ************************************************************************ |
|
| Estimation of the Critical properties. |
|
| ************************************************************************ |
|
|
| Critical Temperature: 677.45 [K] |
|
| Standard Error: Error was not estimated. |
|
| Joback
fragmentation method |
|
|
| Critical
Pressure: 59.263 [bar] |
|
| Standard Error: Error was not estimated. |
|
| Joback
fragmentation method |
|
|
| Critical Volume: 279.50
[cm.cm.cm/mol] |
|
| Standard Error: Error was not estimated. |
|
| Joback
fragmentation method |
|
|
| ************************************************************************ |
|
| Estimation of the Thermodynamics
properties |
|
| ************************************************************************ |
|
|
| Heat of
Formation [T=298.15K, p=1atm]: -96.480 [kJ/mol] |
|
| Standard Error: Error was not estimated. |
|
| Joback
fragmentation method |
|
|
| Gibbs
Energy [T=298.15K, p=1atm]: -32.940 [kJ/mol] |
|
| Standard Error: Error was not estimated. |
|
| Joback
fragmentation method |
|
|
| Ideal gas
thermal capacity for T= 298.15 [K] and p=1atm: 99.577 [J/(mol.K)] |
|
| Standard Error: Error was not estimated. |
|
| Joback
fragmentation method |
|
|
| ----------------------------------------- |
|
|
| ------------ GAMESS Interface
------------ |
|
| GAMESS Job: Compute Properties RHF/3-21G |
|
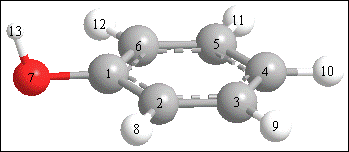
| Charges (Mulliken Charges): |
|
| C(1) 0.326880 |
|
| C(2) -0.236138 |
|
| C(3) -0.239774 |
|
| C(4) -0.240284 |
|
| C(5) -0.239542 |
|
| C(6) -0.237210 |
|
| O(7) -0.738948 |
|
| H(8) 0.247110 |
|
| H(9) 0.241763 |
|
| H(10) 0.237570 |
|
| H(11) 0.241667 |
|
| H(12) 0.246548 |
|
| H(13) 0.390358 |
|
| ------------------------------------------ |
|
|
|
|
| Joback
fragmentation method |
|
|
| Gibbs
Energy [T=298.15K, p=1atm]: -32.940 [kJ/mol] |
|
| Standard Error: Error was not estimated. |
|
| Joback
fragmentation method |
|
|
| Ideal gas
thermal capacity for T= 298.15 [K] and p=1atm: 99.577 [J/(mol.K)] |
|
| Standard Error: Error was not estimated. |
|
| Joback
fragmentation method |
|
|
| ----------------------------------------- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|

